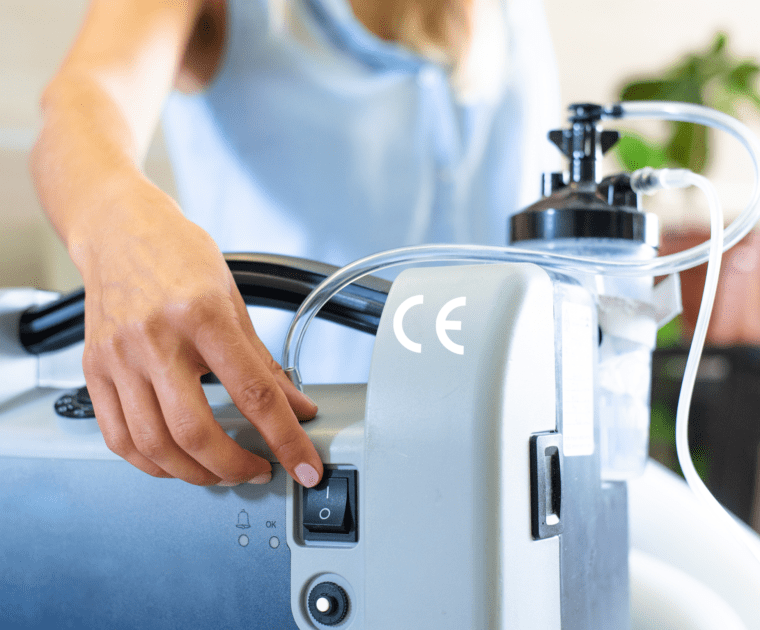
Use of CE marking in the United Kingdom: new extensions for manufacturers
The CE marking proves that goods on the EU market meet the applicable regulatory requirements.…




On April 13, 2022, the United Kingdom notified the draft “The Toys and Cosmetic Products…

Millions of tonnes of plastic waste is produced and imported into the UK every year…

A standard can be understood as a harmonised way of doing something. It usually contains…

CONTEXT: POST-BREXIT COVID-19 UK Taking into consideration the current sanitary situation in Europe and the…
On 28 July 2021, the UK government made it a legal requirement to seek validation…

Recent events show that the UK is determined to provide its RA regime with an…